pH in hydroponic nutrient solutions and its effect on nutrient uptake
What is pH
pH is the measurement of acidity or alkalinity of a solution. It is based on a logarithmic scale and starts at 0 and ends at 14 where the value 7 indicates a neutral, neither acid or alkaline. If a solution has a pH value below 7 it is called an acid. If the solution has a pH value above 7 it is alkaline. The optimum for most vegetables grown in hydroponics is between 5.8 and 6.8. As the pH moves outside the optimum range, nutrients become less available and some may even precipitate which renders them useless for the plant.
If the pH moves from 5 to 6, it is a ten-time shift from being acid to more alkaline (or less acidic). Thus, a full unit shift in pH, away from the optimum range, will result in a significant decrease in nutrient uptake by plant roots. It is thus important to monitor the pH of the nutrient solution and maintain it within the optimum range.
How to measure pH in hydroponic nutrient solutions
Acidity or alkalinity can be measured in various ways. The old method is through litmus paper which tests between 4.0 and 7.0 in 0.3 to 0.4 increments. The litmus paper colour changes from yellow (4.0) to dark blue (7.0).
Another method is to use liquid indicators. These types are commonly used with swimming pools. The liquid changes depending how acid or alkaline the nutrient solution is. A sample of the nutrient solution is placed in a test tube. A drop or a small pellet is placed in the tube and shaken for a minute. The colour of the liquid is measured against a colour card which will indicate the pH.
The easiest method is to obtain an electronic pH meter (Such as the Oakton EcoTestr pH 2 Waterproof pH Tester, 0.0 to 14.0 pH Range) which is the most accurate to use. Some can measure pH and electrical conductivity (EC). It is important to have a hand held pH and EC meter even though an automated system is installed. Even the best automated control equipment can develop faults and independent measuring is necessary.
pH and plant nutrition
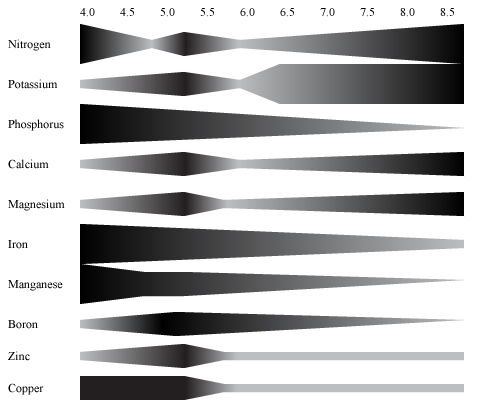
The reason why it is important to maintain a pH between 5.8 and 6.8 is illustrated in the figure below. It is evident that the elements availability varies as pH changes. Critical elements are phosphorus, boron and manganese. Their availability decreases drastically below 6.0. Magnesium and calcium show opposite reactions, at acidic values their availability is restricted but increase as the nutrient solution becomes more alkaline increases.
Share This Story, Choose Your Platform!
Cotton aphid – Aphis gossypii
Cotton Aphid, or Aphis gossypii, is one of the more common insect pests found in almost all vegetable crops and [...]
Greenhouse white fly
Description Greenhouse white fly, also known as Trialeurodes vaporariorum is a sap feeder. The easiest way to find white flies [...]